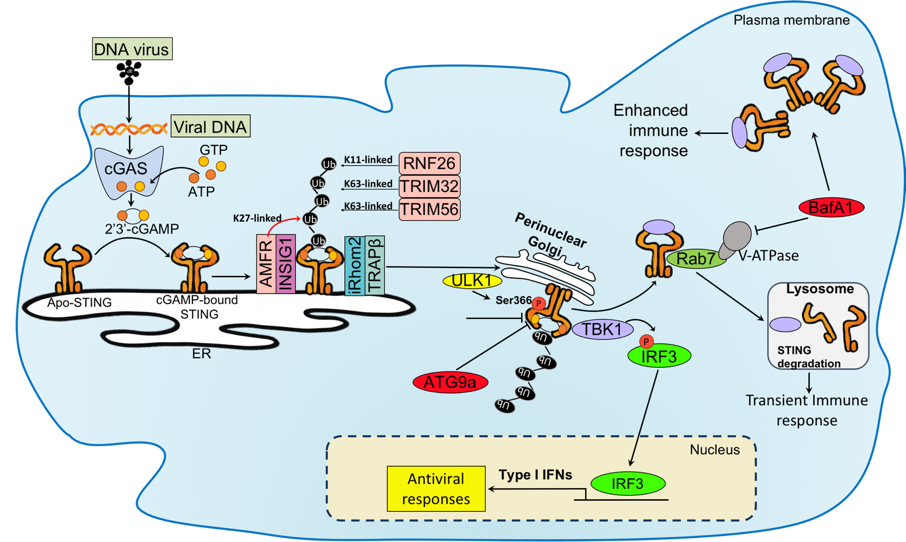
Various regulatory mechanisms for cGAS and STING pathway have been shown (Cai et al., 2014; Chen and Chen, 2016; Liu et at., 2015; Liu and Wang 2016; Hu et al., 2016). Sumoylation and Mono-ubiquitination of cGAS at Lys355 that are induced by E3 ubiquitin ligase TRIM56 is important for its DNA sensing activity, resulting in increased cGAMP production (Xiong et al., 2018; Sun et al., 2013; Seo et al., 2018). STING moves from ER to Golgi apparatus via the translocon-associated protein (TRAP) complex TRAPb(SSR2) that is recruited by inactive rhomboid protein 2 (iRhom2) and these complex reaches to Sec-5 containing perinuclear microsome or cytoplasmic punctate structures to assemble with TBK1 and IKK complex (Ishikawa et al., 2008; Abe and Barber, 2014; Luo et al, 2016). RAB2B-GARIL5 (Golgi-associated RAB2B interactor-like 5) complex is a regulator of STING in Golgi apparatus and promotes IFN response through regulating phosphorylation of IRF3 by TBK1. The autophagy-related protein such as autophagy-related protein (Atg), ULK1 (a homologue of Atg1), microtubule-associated protein 1 light chain (LC)3 (homologue of yeast Atg8) and Atg9 negatively regulate STING signaling through interfering with STING-TBK1-IRF3 signaling (Saitoh et al., 2009, Tooze et al., 2010; Konno et al., 2013). Atg-mediated degradation modulates baseline of STING protein level, but it does not impact the trafficking of STING. The function of STING is regulated by post-translational mechanism such as TRIM32 and TRIM56, that conjugate K63-linked polyubiquitination on STING and promote the recruitment of TBK1 (Tsuchida et al., 2010). ER-associated E3 ligase, AMFR, catalyzes the K27-linked polyubiquitination of STING together with INSIG1 (Wang et al., 2014). K27-linked polyubiquitination on STING induces TBK1 recruitment and activation. iRhom2, which recruits de-ubiquitination enzyme EIF3S, maintains the stability STING through removal of its K48-linked polyubiquitin chains. STING translocates from ER to Golgi apparatus and then moves to late endosome/lysosome (Dobbs et al., 2015). Helix aa281-297 of STING is shown to be degraded through V-ATPase in endosome/lysosome. The blockade of V-ATPase suppresses STING degradation, which potentially leads to enhance STING signaling (Gonugunta et al., 2017).
onse through regulating phosphorylation of IRF3 by TBK1. The autophagy-related protein such as autophagy-related protein (Atg), ULK1 (a homologue of Atg1), microtubule-associated protein 1 light chain (LC)3 (homologue of yeast Atg8) and Atg9 negatively regulate STING signaling through interfering with STING-TBK1-IRF3 signaling (Saitoh et al., 2009, Tooze et al., 2010; Konno et al., 2013). Atg-mediated degradation modulates baseline of STING protein level, but it does not impact the trafficking of STING. The function of STING is regulated by post-translational mechanism such as TRIM32 and TRIM56, that conjugate K63-linked polyubiquitination on STING and promote the recruitment of TBK1 (Tsuchida et al., 2010). ER-associated E3 ligase, AMFR, catalyzes the K27-linked polyubiquitination of STING together with INSIG1 (Wang et al., 2014). K27-linked polyubiquitination on STING induces TBK1 recruitment and activation. iRhom2, which recruits de-ubiquitination enzyme EIF3S, maintains the stability STING through removal of its K48-linked polyubiquitin chains. STING translocates from ER to Golgi apparatus and then moves to late endosome/lysosome (Dobbs et al., 2015). Helix aa281-297 of STING is shown to be degraded through V-ATPase in endosome/lysosome. The blockade of V-ATPase suppresses STING degradation, which potentially leads to enhance STING signaling (Gonugunta et al., 2017).
references :
Cai, X., Chiu, Y. H., & Chen, Z. J. (2014). The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Molecular cell, 54(2), 289-296.
Chen, Q., Sun, L., & Chen, Z. J. (2016). Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nature immunology, 17(10), 1142.
Liu, X., & Wang, C. (2016). The emerging roles of the STING adaptor protein in immunity and diseases. Immunology, 147(3), 285-291.
Hu, M.M., Yang, Q., Xie, X.Q., Liao, C.Y., Lin, H., Liu, T.T., Yin, L. and Shu, H.B. (2016). Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity, 45(3), pp.555-569.
Xiong, M., Wang, S., Wang, Y. Y., & Ran, Y. (2018). The Regulation of cGAS. Virologica Sinica, 1-8.
Sun, L., Wu, J., Du, F., Chen, X., & Chen, Z. J. (2013). Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science, 339(6121), 786-791.
Seo, Gil Ju, et al. “TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing.” Nature communications 9.1 (2018): 613.
Ishikawa, H., & Barber, G. N. (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature, 455(7213), 674.
Abe, T., & Barber, G. N. (2014). Cytosolic DNA-Mediated, STING-Dependent Pro-Inflammatory Gene Induction Necessitates canonical NF-κΒactivation Through TBK1. Journal of virology, JVI-00037
Luo, W. W., Li, S., Li, C., Lian, H., Yang, Q., Zhong, B., & Shu, H. B. (2016). iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nature immunology, 17(9), 1057.
Saitoh, T., Fujita, N., Hayashi, T., Takahara, K., Satoh, T., Lee, H., Matsunaga, K., Kageyama, S., Omori, H., Noda, T. and Yamamoto, N., (2009). Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proceedings of the National Academy of Sciences, 106(49), pp.20842-20846.
Tooze, S.A., Jefferies, H.B., Kalie, E., Longatti, A., Mcalpine, F.E., Mcknight, N.C., Orsi, A., Polson, H.E., Razi, M., Robinson, D.J. and Webber, J.L., (2010). Trafficking and signaling in mammalian autophagy. IUBMB life, 62(7), pp.503-508.
Konno, H., Konno, K., & Barber, G. N. (2013). Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell, 155(3), 688-698.
Tsuchida, T., Zou, J., Saitoh, T., Kumar, H., Abe, T., Matsuura, Y., Kawai, T. and Akira, S., (2010). The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity, 33(5), pp.765-776.
Wang, Q., Liu, X., Cui, Y., Tang, Y., Chen, W., Li, S., Yu, H., Pan, Y. and Wang, C., (2014). The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity, 41(6), pp.919-933.
Dobbs, N., Burnaevskiy, N., Chen, D., Gonugunta, V. K., Alto, N. M., & Yan, N. (2015). STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell host & microbe, 18(2), 157-168.
Gonugunta, V. K., Sakai, T., Pokatayev, V., Yang, K., Wu, J., Dobbs, N., & Yan, N. (2017). Trafficking-Mediated STING Degradation Requires Sorting to Acidified Endolysosomes and Can Be Targeted to Enhance Anti-tumor Response. Cell reports, 21(11), 3234-3242.